![]()
2023-06-21 updated
CYBERDYNE Inc. (Head office: Tsukuba, Ibaraki, Japan, President & CEO: Yoshiyuki Sankai, from now on referred to as the “Company”) announces that it has applied the Pharmaceuticals and Medical Devices Agency (“PMDA”) for a manufacturing and marketing approval for the newly developed small model of the Medical HAL Lower Limb Type (from now on referred to as “Medical HAL”) as a medical device for previously approved applicable diseases*1 in Japan.
The new small model is designed for patients with a body size below the applicable range of existing models already used as medical devices. The development of this device was partially supported by Japan Agency for Medical Research and Development (“AMED”) *2.
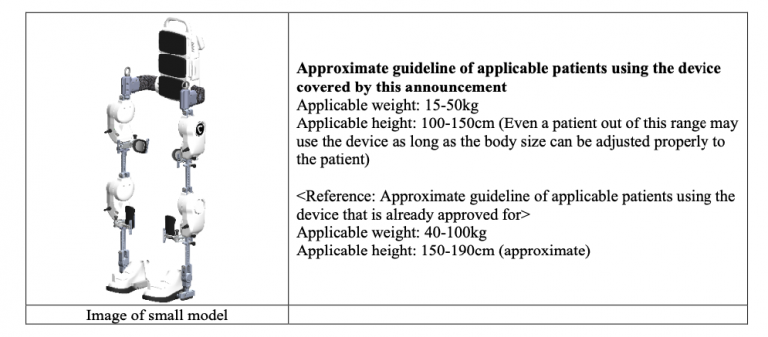
This application for approval is expected to be a significant step forward in expanding treatment opportunities with Medical HAL for small patients who have been unable to use Medical HAL due to size incompatibility. The Company will make every effort to submit information to PMDA so that the application can proceed smoothly.
In addition, the Company will progressively apply for medical device applications for this smaller model in Europe, the U.S., Asia, and other foreign countries.
*1 Target disease of Medical HAL that PMDA already approves:
Neuromuscular Disease (8 conditions)
Spinal Muscular Atrophy (SMA), Spinal and Bulbar Muscular Atrophy (SBMA), Amyotrophic Lateral Sclerosis (ALS), Charcot-Marie-Tooth disease (CMT), Distal Myopathy, Inclusion Body Myositis (IBM), Congenital Myopathy, Muscular Dystrophy
Spinal disorder (2 conditions)
HTLV-1 associated myelopathy (HAM), Hereditary Spastic Paraplegia
*2 Support from AMED
In the related AMED program for the fiscal year 2019 to 2023, “clinical trial/clinical research to actualize a medical device for children,” “Project to develop and conduct an investigator-initiated clinical trial of wearable treatment robot for lower limb designed to improve the motor function of children with developmental stage non-progressive motor dysfunction such as cerebral palsy” (Lead investigator: Dr. Aiki Marushima of Tsukuba University) is currently underway. The pediatric lower limb-mounted treatment robot developed in this research activity for the above clinical trial is equivalent to the products announced by this release.
In the reporting session of the project, the Company received comments that medical device for pediatrics is often faced with the problem of profitability. However, the Company aims to solve this problem by adjusting the range of applicable patients to adults with smaller body sizes and extending the application to target diseases already approved for HAL.
© 2026 CYBERDYNE INC. ALL RIGHTS RESERVED.